STARS Curricula
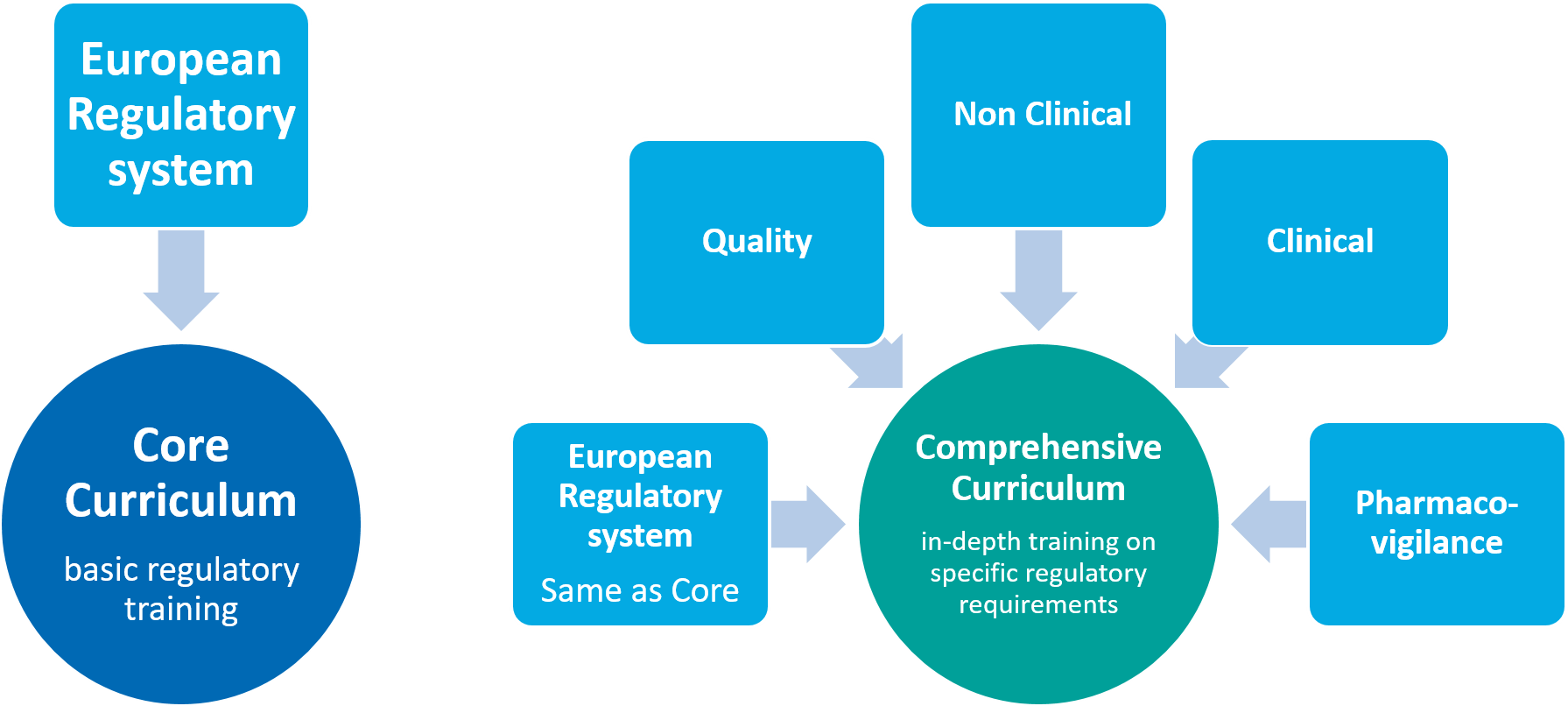
To overcome the regulatory knowledge gaps, the STARS consortium developed a curriculum to strengthen the awareness of regulatory science in academia, on the basis of survey data and the outcomes of interactive multi-stakeholder workshops. The focus was on the development of a Core Curriculum (CoC) dedicated to basic regulatory training, and a Comprehensive Curriculum (CpC) focused on a more indepth training on specific regulatory requirements. The curricula concept has been conceived as guidance to achieve a harmonised and common level of regulatory knowledge in academia in the near future.
Disclaimer: The STARS consortium is not teaching the curricula. The curricula are considered as a superordinate recommendation for an EU-harmonized concept. It is addressed to universities or post-graduate courses in the EU member states. The STARS curricula do not aim to substitute or replace any existing European curricula, and the curricula can be adapted to the different needs and requirements of the respective national education systems of the European member states.
STARS Core Curriculum
The Core Curriculum is mainly targeted at graduate students (bachelor and master’s degree) interested in regulatory science and in gaining basic knowledge/training of European regulations on medicinal products and borderline between medicines and medical devices. This Core Curriculum aims to provide attendees an overview of regulatory science and the regulatory system in Europe, giving an overview of development pathways, the EU legislation and the use of guidelines, with an introduction on the core parts of a clinical trial application and marketing authorization application (quality, non-clinical and clinical) and on the post-marketing processes.

For more information on the content of the Core Curriculum modules and learning outcomes, please download the accompanying document:
STARS Comprehensive Curriculum
With the rapid expansion of pharmaceutical and biomedical products and increasing complexity of innovative technologies and products, more highly skilled professionals who have the expertise to conduct research in compliance with complex regulatory policies and challenging procedures are needed. The Comprehensive Curriculum is designed for an advanced training level to acquire more in-depth knowledge in regulatory science and to gain more information on different and especially innovative regulatory areas with the overarching goal to successfully develop novel medicinal products and technologies for patients. The target audiences are researchers and healthcare professionals involved in medicinal development.
The Comprehensive Curriculum t is divided into five different modules. They will provide an overview of legislation, tools, approaches, standards and latest guidelines that are essential to develop innovative medicinal products with the required level of quality, safety, and efficacy to be marketed within the EU. This specialised education is crucial for professionals to develop a comprehensive understanding of the appropriate regulatory requirements related to their specific field of interest and of the timely use of the NCA support activities during the product development. Hence, professionals will be trained to identify and interpret the specific regulatory framework that will be crucial in driving forward their research to develop a new product.
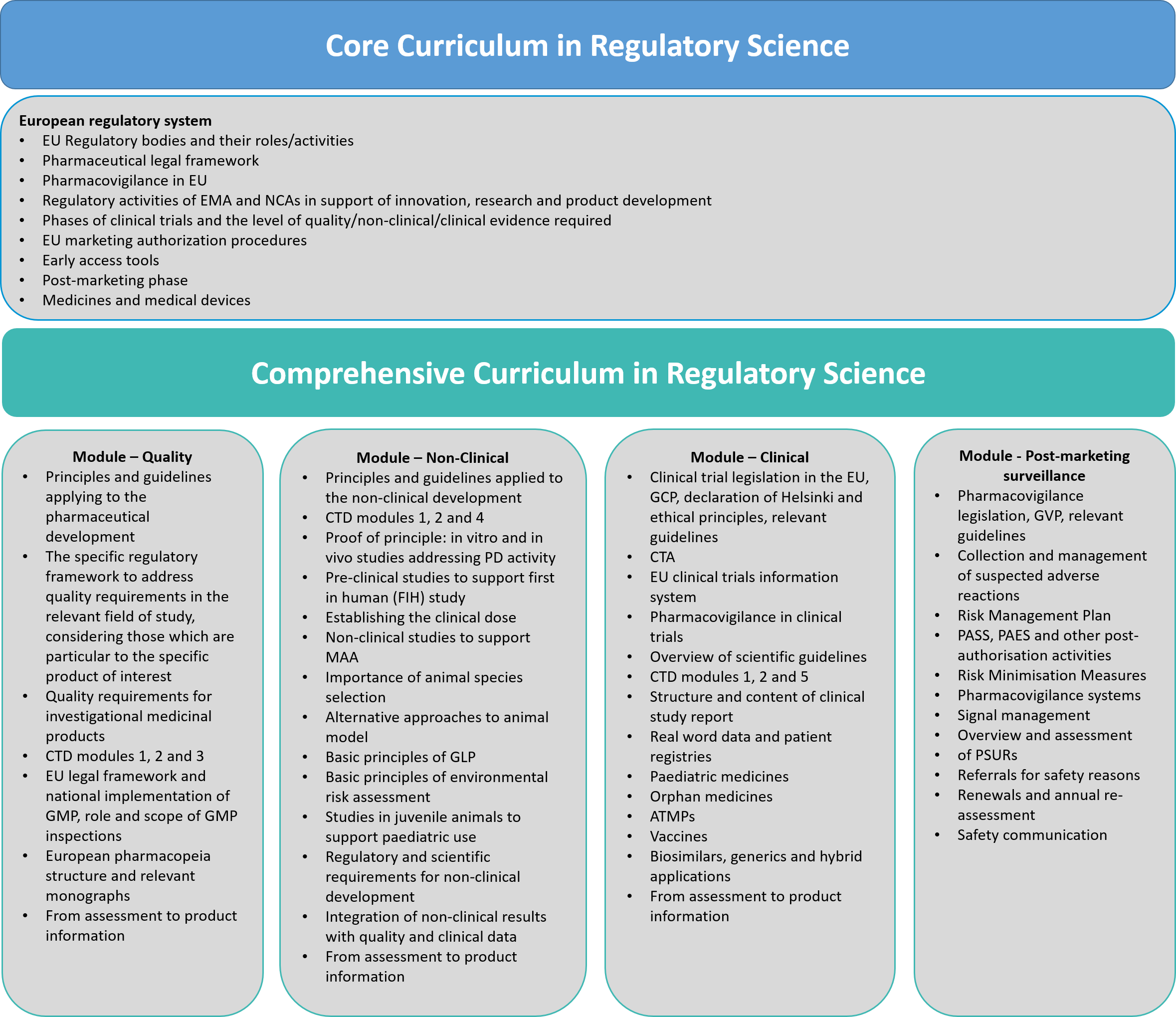
For more information on the content of the Comprehensive Curriculum modules and learning outcomes, please download the accompanying document:
