STARS Pilots and Training Materials
In order to provide a proof-of-concept and to demonstrate that selected support activities and elements work efficiently, the STARS consortium has performed three pilots.

Further details on the implementation of the individual pilots, as well as their results and evaluations, can be found in Chapter 4 of the STARS Common Strategy under 'STARS Pilots'.
Below, you will find an overview of the training materials developed in the course of the pilots.
Pilot I - Best Practice Transfer
The aim of Pilot I was to transfer an identified best-practice example in the EU to another EEA member state. This led to the design of an online training course in regulatory science for academic researchers.
Title of the course: The Winding Road from a Brilliant Idea to Drug Approval: An Online Course in Regulatory Science for Academic Researchers
Objectives of the training course:
- to cover clinical, non-clinical and quality aspects relating to all important steps in drug development – from laboratory practice to clinical applications
- to introduce national and European regulatory frameworks and give an overview of regulatory support tools for academia
- to give the opportunity to interact with regulators and ask questions.
Training material (course presentations)
Pilot II - Addressing gaps: initiation of a novel support activity for improving success on regulatory Scientific Advice
The objective of the Pilot II was to identify a gap which required the establishment of a new support activity with a substantial impact on academic driven health research and a potential to provide benefit for patients.
The development of this novel support activity was based on comprehensive survey data that were collected within the course of the STARS project. We surveyed a wide scope of stakeholders regarding different aspects on regulatory awareness, knowledge, and support. In total, we analysed data from 449 academic health research groups, 88 health research centres, 40 funding bodies and 21 NCAs with a view to best practices and gaps in the level of regulatory awareness, knowledge, attitude and approaches in relation to academic clinical research and regulatory science.
Comprehensive data analysis revealed that there the academic community has a requirement for tools to improve the communication between regulators and academia, and that a timely response is probably the most remarkable need.
Based on these findings, Pilot II was developed as a one-stop-shop platform that enables academic researchers to access general content on regulatory aspects but also ask some quick questions.
From 1-30 September 2021, we temporarily opened a one-stop-shop communication platform for Spanish academia exclusively.
An integral component of the one-stop shop was the so-called Information Board, which contained the following important regulatory documents and guidelines. Please note that many components or linked websites are in Spanish, as Pilot 2 was conducted in Spain, as described above.
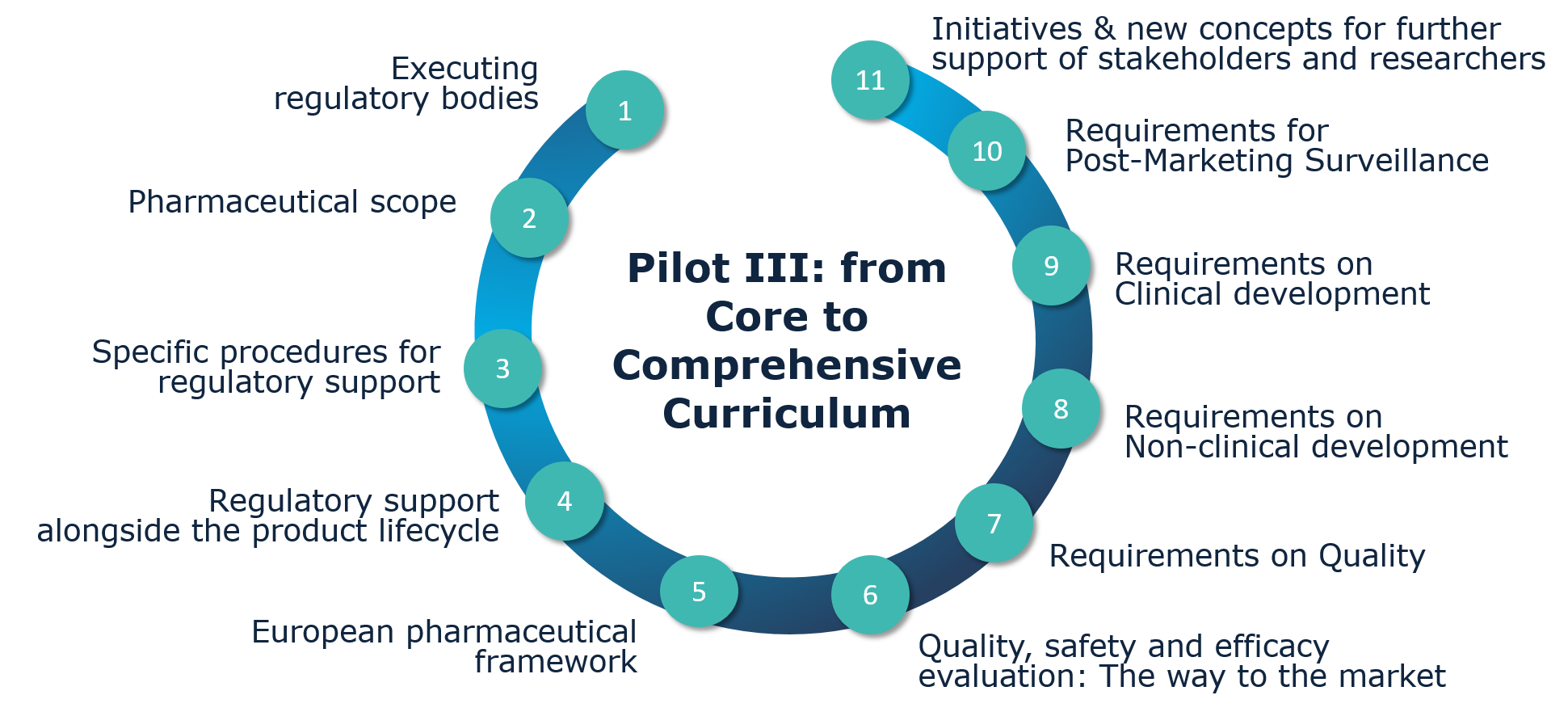
Pilot III - Implementing the Comprehensive Curriculum
Pilot III represents the first approach towards implementing the STARS Comprehensive Curriculum for strengthening regulatory knowledge in academia. The format of this pilot was conceived as an online training programme to provide key entrance for clinical researchers and scientists at different levels of education and regulatory knowledge. They were introduced into the legal framework and scientific guidance and also could learn about the timely use of the NCA support activities during the development of their products.
The online training programme called “Regulatory Support to Spanish Academia from STARS Core to Comprehensive Curriculum” consisted of the STARS Core Curriculum with some information from the other four modules included in the STARS Comprehensive Curriculum (quality module, non-clinical module, clinical module and post-marketing surveillance module).