Non-eCTD electronic Submission (NeeS)
Attention
Since 01.01.2019, non-eCTD-submissions (NeeS) may only be submitted for parallel imports.
Companies must ensure that the sequences in all submissions, for which only the eCTD-format is permitted, are submitted in a technical valid eCTD-format.
If a dossier has been maintained in its lifecycle in the non-eCTD-format, it is permitted to conduct a change of the format from non-eCTD to eCTD without a prior announcement.
For this purpose, it is necessary to provide a baseline submission in eCTD-format starting with the sequence 0000 (module 1 to module 3).
The following information should be included in the cover letter of the baseline submission:
- Reason for the baseline submission: change of the format (non-eCTD to eCTD)
- A confirmation that the content of the baseline is identical with the already existing documents resp. that no changes in the content have been conducted.
BfArM will accept electronic submission following the requirements set out in the NeeS-Guideline, without the XML backbone of the eCTD format. Detailed information about structuring and formatting of Non-eCTD electronic Submissions (NeeS) is available at the website of the eSubmission Change Management Boards (Sub CMB).
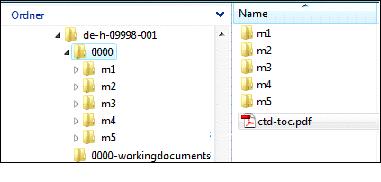
If a submission concerns multiple applications or authorised products (e.g. several ENRs) the respective folders have to be created named by <procedure number.> or <ENR> (e.g. de-h-09999-001, de-h-09999-002 etc.).

Below this, the root directory with the sequence number should be created. Within the sequence, the folders for the modules and the table of contents “ctd-toc.pdf” must be present. Analog to eCTD-format, working documents as office-documents (preferably in docx-format; doc-format is currently still acceptable), that comply with the documents specified in the AMG submission ordinance (AMG-EV) for minimum electronic submission (module 1.3.1: 131-spclabelpl), must be submitted in a separate folder “<sequence>-workingdocuments” via CESP or on the same data storage device.
Prior to uploading the sequence via Common European Submission Portal (CESP) or sending the data storage device it has to be ensured that the data can be read correctly (e.g. CRC errors, which may occur during the copying process, must be ruled out).
For technical validation, ensure prior to uploading via CESP or burning the data to the data storage device that the sequence is identical with the documentation to be sent to BfArM.
BfArM does not require a report on technical validation; however, you should have the report available in case questions are raised.
