#5 Tips for DiGA applicants
BlogTesaser_Stand
We know how to DiGA: Balance sheet for the directory anniversary
0 consultations for DiGA were held at the BfArM within approximately one year.
For more than a year now, physicians and psychotherapists in Germany have been able to prescribe medical apps and other Digital Health Applications (DiGA). If the diagnosis is appropriate, reimbursement directly from the statutory health insurance is also possible - a development in which the BfArM was and is significantly involved.
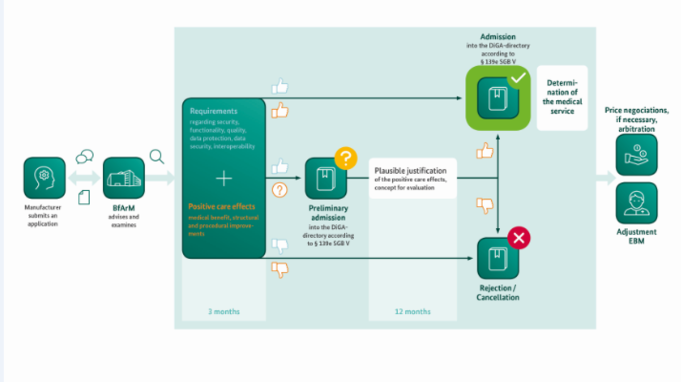
For patients to be able to use such applications quickly and safely, a new path to reimbursement was created for manufacturers: the so-called DiGA fast-track procedure. Within a three-month period starting with the filing of the complete application, the BfArM assesses the DiGA in question. The essence of this assessment includes the examination of the manufacturer’s statements regarding the properties of the product - from data protection to user-friendliness – as well as consideration of the evidence of the DiGA’s positive healthcare effect as provided by the manufacturer. After successful evaluation, the product can then be listed in the BfArM’s DiGA directory which in turn is the prerequisite for its prescription and reimbursement.
Fast Track is also a model for other countries
Germany has successfully broken new ground with this procedure – meanwhile, the DiGA fast-track procedure is also being used in other countries as a model for similar procedures.
The number of consultations at the BfArM shows that the interest of manufacturers is great. In just over a year, about 230 discussions were held with manufacturers, mainly on procedural details and the evidence to be provided. In addition, there were more than 560 general enquiries regarding the fast-track procedure itself.
A total of 97 applications for listing of a DiGA have already been submitted to the BfArM. Today, 22 apps are currently listed in the directory, including, for example, apps that are intended to support the treatment of anxiety disorders, migraine or sleep deficiencies.
It is in the BfArM’s best interest that manufacturers pass through the DiGA fast-track procedure successfully. Applicants therefore have many opportunities to seek help around the assessment process. Important questions can be clarified in advance: How should relevant documents be compiled? What data is needed for (final) listing in the directory? Experts are available to answer these questions in a so-called "kick-off meeting". These meetings are offered by the BfArM Innovation Office, which was set up in 2017 as a straightforward first point of contact for start-ups, researchers and developers.
We want manufacturers to go through the process successfully
The head of the Innovation Office is Dr Wiebke Löbker. Together with her team and colleagues from the scientific departments, she has so far conducted about 230 consultations on DiGAs alone. "An exciting task from which not only the applicants benefit, but we as well," she explains. She goes on to say that much of the feedback gained during the process has already been incorporated into legislature's catalogue of requirements. "Together we are working on improving patient care. Therefore, we all have an interest in the manufacturers being able to list their applications in the directory in the end."
In fact, the comparatively large number of applications withdrawn by manufacturers shows that there is often still need for improvement in one place or another. The head of the Innovation Office offers the following tips for a successful DiGA submission:
#1 Talking helps: Take advantage of the BfArM's advisory services before submitting an application.
Our experience shows: We see a significantly higher overall quality in the submitted documents by applicants who have sought advice from the BfArM early on before making a submission. We can therefore only recommend this: Ask us, come to the counselling interview, so that together we can address possible challenges in advance of the submission! This is particularly important in view of the deadlines in the DiGA fast-track procedure, because this gives you the opportunity to close any gaps, which sometimes only become apparent in the dialogue, even before the procedure starts. Most of the manufacturers who withdrew their application stated that they needed more time to prepare and would then renew their submission. Here, too, the BfArM offers optimal support: Those who are preparing to resubmit their documents can also receive extensive advice from us - on all aspects of the procedure.
#2 Keeping an eye on deadlines - the clock is ticking
The fast-track procedure takes a maximum of three months. Unlike the marketing authorisation procedures for medicinal products, it also does not offer the possibility of a so-called "clock-stop". This means that the deadlines can no longer be stopped in the ongoing procedure which can become a challenge - for example, if it only becomes clear during the process that data or other information must be supplemented. Even in such cases, manufacturers have to meet the deadlines. Let's take the example of a systematic data evaluation that turns out not to be significant enough. This creates uncertainties regarding the evidence and it is obvious that it will not be possible to produce such data within a short period of time.
If the manufacturer cannot remedy such deficiencies within the binding assessment period, there is no possibility for the BfArM to include this DiGA in the directory - not even as a preliminary listing. All the more important therefore: See tip #1.
#3 Importance of evidence: Immense
Our experience from more than one year of DiGA shows: Especially in terms of evidence, the quality of the submitted documentation varies considerably.
Initially there is the question as to the type of submission: is the DiGA intended for a preliminary or a final listing in the directory?
In the case of a preliminary inclusion for testing, the systematic data evaluation plays a central role. It represents the plausible justification for the improvement of care. The evaluation concepts can usually be adapted at short notice - i.e. even during the ongoing evaluation procedure.
We have observed various problems with these submissions: For example, observation periods were clearly too short for a systematic data evaluation. Or positive healthcare effects were claimed, which, however, were not addressed by the data evaluation. Furthermore, subject numbers in the lower single-digit range are clearly insufficient.
Another reason that led to the withdrawal of applications for preliminary inclusion: the DiGAV stipulates that the systematic data evaluation must be based on surveys with the DiGA itself. Simply referring to other DiGAs or publications is not sufficient in this context.
A central problem in connection with applications for final inclusion is that older studies were not designed for the purpose of a health technology assessment. They had considerable limitations or could not show confirmatory evidence. For example, documents such as the study protocol, which provide important information e.g. on prespecification, were missing.
These are all aspects that we can discuss with the manufacturers in advance, giving them time to prepare their application in the best possible way.
#4 Studies: RCTs are usually the most suitable, especially when it comes to medical benefits.
In order to prove a positive healthcare effect, a retrospective comparison is generally the minimum requirement for a DiGA. For the DiGAs currently listed in the directory, randomised controlled trials (RCTs) were submitted exclusively, or at least as the "primary" data basis for evaluation.
This picture is further confirmed in consultations: in most cases, RCTs are the design best suited for the planned evidence and therefore also directly intended by the manufacturers from the outset.
What we have rarely seen so far are retrospective data evaluations. However, the DiGAV clearly states that these are possible if the hypotheses can be confirmed. In principle, healthcare data can be used in various constellations, for example as historical control groups.
Intra-individual comparisons can also be used in the submission. However, it must be ensured that the effect is actually attributable to the DiGA and not, for example, to seasonal effects in the case of an allergy app.
For proof of a medical benefit, however, we actually rather tend to see RCTs so far. We assume that in the future, fast-track evaluations will also be increasingly based on healthcare data from "real healthcare contexts”, especially when it comes to evidence of procedural and structural improvements.
#5 Think holistically - also focus on interoperability, accessibility and user-friendliness
Interoperability parameters are important aspects that should be borne in mind, from the initial idea to the practical implementation - in addition to the positive supply/care? effects, data protection and information security. After all, DiGA should not be viewed in isolation, but should be integrated seamlessly into the increasingly networked digital ecosystem; they should be able to interact not only with hardware components such as blood glucose meters or the like, but also with electronic patient files, for example. Accessibility and usability are of great importance for users and patients – the relevance this has with regard to DiGA is set out on the one hand in general terms in the DiGA guidelines. What this means in individual cases for a specific DiGA on the other hand is something that the manufacturers can discuss with us in concrete terms - see Tip #1 .
Advancing innovations, supporting manufacturers, accompanying developments: The Innovation Office provides assistance in preparing for DiGA submissions and supports manufacturers with advice and assistance: www.bfarm.de/Innovation
For more than a year now, it has been possible to make submissions for the inclusion of digital health applications in the DiGA directory to the BfArM. The fast-track procedure is a key contribution to the rapid and quality-assured establishment of digital apps in Germany: www.bfarm.de/diga
Shaping the digitalisation of healthcare together: How BfArM is actively driving this process in Germany and Europe and the role of the Innovation Office: www.bfarm.de/digitalfuture
Dr. Wiebke Löbker

University studies of Pharmacy. From 2009 to 2011, research assistant at the Institute of Pharmacology and Toxicology at the Free University of Berlin. From 2011 to 2016, consultant and team coordinator in the Pharmaceuticals Department of the Federal Joint Committee, focusing on early benefit assessments. Has been working at the BfArM as head of the executive department “Innovation Office/Change Management” and as personal assistant to the President since 2016.
